Case Study – Part 2
You ordered a CXR and spirometry at the previous visit and he returns today to review the results. Physical exam and symptoms are unchanged since last visit. Vital signs at this visit are: Temp-98.3, P-68, RR-20, BP 152/90, Height 68.9in., Weight 258 pounds, O2sat 94% on RA
CXR Result:
No acute infiltrates or consolidations are seen. Cardiac and mediastinal silhouettes are normal. No hilar enlargement is evident. Osseous thorax is intact.
Spirometry Results:
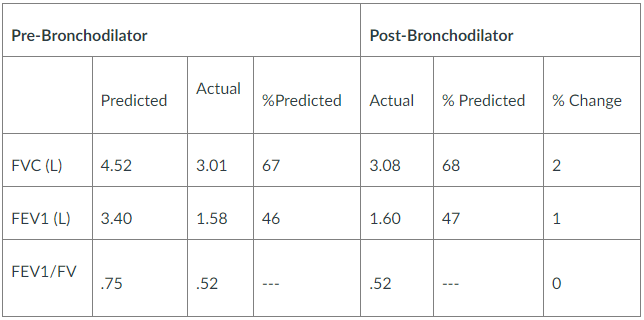
Requirements/Questions:
- What is your primary (one) diagnosis for this patient at this time? (support the decision for your diagnosis with pertinent positives and negatives from the case)
- Identify the corresponding ICD-10 code.
- Provide a treatment plan for this patient’s primary diagnosis which includes:
- Medication*
- Any additional testing necessary for this particular diagnosis*
- Patient education
- Referral
- Follow up
- Provide an active problem list for this patient based on the information given in the case.
- Are there any changes that you would also make to this patient’s overall treatment plan at this time? Must provide an EBP argument for each treatment or testing decision.
*If part of the plan does not warrant an action, you must explain why. ALL medication and testing decisions (or decisions not to treat with medication or additional testing) MUST be supported with an evidence-based practice (EBP) argument. Over-the-counter (OTC) and RXs must be written in full as if handing a script to the patient in the office.
Over-the-counter (OTC) and RXs must be written in full as if handing a prescription to the patient in the office.
Example:
Amoxicillin 500 mg capsule
1 tab po BID q 10 days
Disp #20 no refills
You should also look at (Sample Discussion) NR601 Week 3: Psychiatric Disorders and Screening
Solution-COPD Case Study Part 2
Primary diagnosis
As supported by the Tests indicated previously the patient is suffering from Chronic Obstructive Pulmonary Disease (COPD). The pertinent positives from the investigations that support the diagnosis of COPD are the Prebronchodilator FEV1/FVC ration of 0.52 and the post bronchodilator FEV1/FVC ration of 0.52. According to the Global Initiative for chronic obstructive lung disease (GOLD) criteria a post bronchodilator FEV1/FVC ratio of 0.70 is considered as the diagnostic for COPD (Marçôa et al., 2017). After a diagnosis of the COPD, the GOLD system further categorizes airflow limitation into stages. The patient is on a severe airway limitation (GOLD 3 stage) after results of the predicted FEV1 lies between 30% and 50% (46%). Other positives present with this patient include a previous history of smoking and age….Kindly click the purchase icon to purchase the full solution at $10

